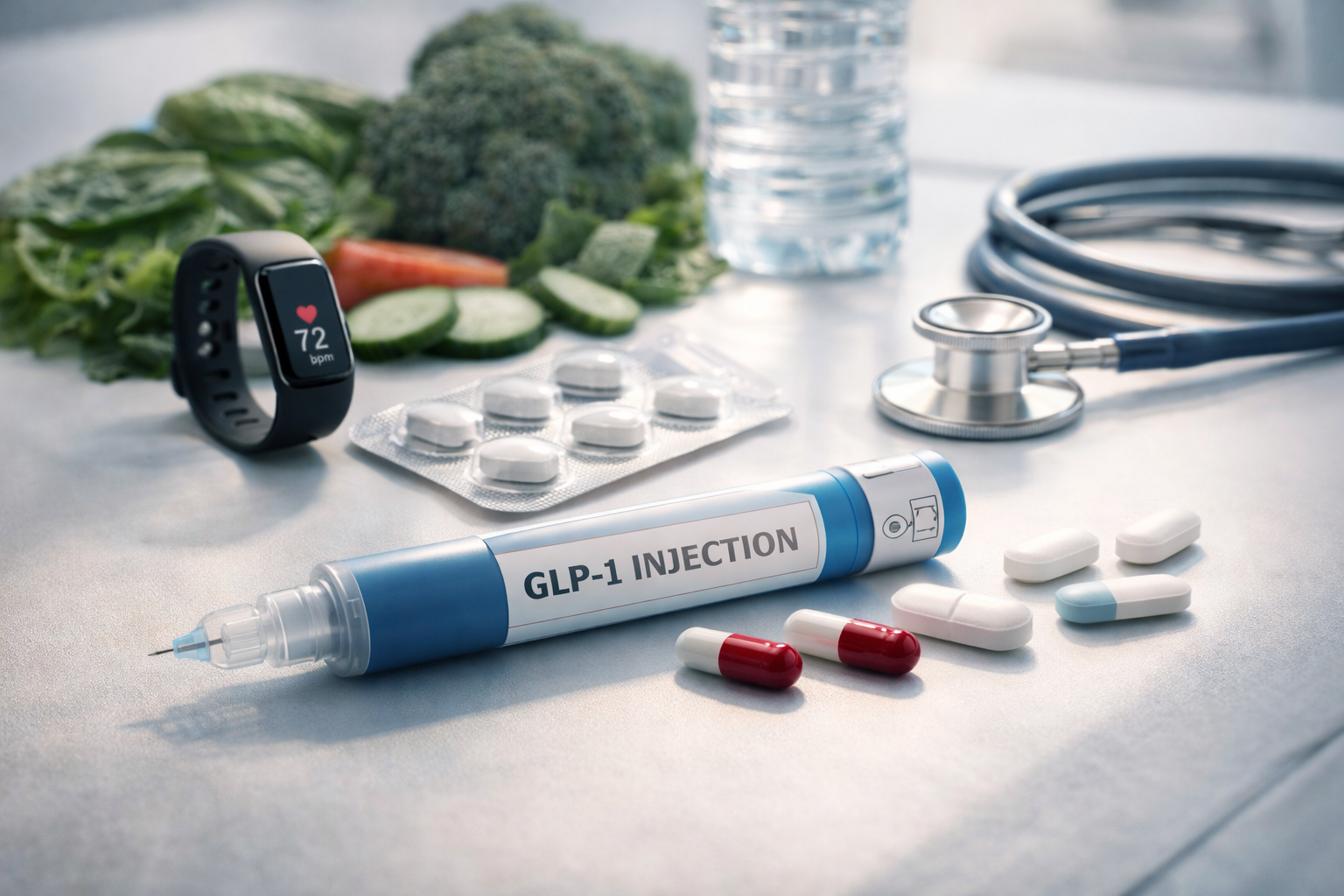
GLP-1 weight loss drugs are transforming healthcare in 2026, with weight-loss injection use expected to double and 3.3 million UK adults likely to use them. The introduction of oral GLP-1 pills marks a pivotal moment in weight management, as these medications move beyond injections to offer more convenient daily options that deliver comparable results.npr+2
The Breakthrough: Oral GLP-1 Pills Arrive
On December 22, 2025, the FDA approved the first oral GLP-1 medication specifically for obesity—the Wegovy pill from Novo Nordisk. This pill contains the same active ingredient as Ozempic and Wegovy injections (semaglutide) and research indicates it is similarly effective for weight loss as the injectable forms. The oral Wegovy became available in early January 2026 at pharmacies and through select telehealth providers, with the starting dose (7 mg) priced at $149/month for cash-pay patients.finance.yahoo+1
A competing oral medication from Eli Lilly is expected to receive approval later in 2026, further expanding access to these transformative treatments. In December 2025, Eli Lilly disclosed that a Phase 3 clinical trial revealed patients receiving the highest dose of their experimental drug lost nearly 29% of their body weight on average after approximately 16 months—an unprecedented result for any GLP-1 medication currently available.nbcnews+1
How GLP-1 Medications Work
GLP-1 (glucagon-like peptide-1) agonists are medications originally developed for type 2 diabetes that have revolutionized weight management. These drugs work by regulating appetite through hormonal pathways, promoting weight reduction when combined with lifestyle changes and medical supervision, and providing metabolic enhancements in many individuals. In early clinical trials, patients on GLP-1 agonists achieved weight loss of 15-20%, compared to the 5-10% typically seen with previous medications.ucdavis+1
The most common GLP-1 medications include semaglutide (marketed as Ozempic for diabetes and Wegovy for weight loss) and tirzepatide (marketed as Mounjaro for diabetes and Zepbound for weight loss). Tirzepatide is actually a dual agonist, acting on both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors, which may contribute to superior weight-loss outcomes.medscape+2
Clinical Effectiveness and Benefits
Weight Loss Results
GLP-1 agonists have achieved dramatic weight loss results that were previously unimaginable. These medications are transforming the treatment landscape, dramatically improving the amount of weight loss achievable through medication alone or in combination with lifestyle interventions. Clinical trials have demonstrated that tirzepatide shows superior weight-loss outcomes compared to semaglutide alone.healthline+3
Advanced medications in development, such as retatrutide, have shown even more impressive results, helping patients lose 58 pounds on average in less time than semaglutide or tirzepatide. This represents an important leap forward that could fundamentally change how severe obesity is approached.medscape
Beyond Weight Loss: Systemic Health Benefits
GLP-1 agonists have demonstrated abilities far beyond glycemic control and weight reduction. These medications reduce major cardiovascular events including heart attack and stroke, as GLP-1 receptors in the heart and blood vessels influence blood pressure, lipid profiles, and inflammation. They also combat fatty liver disease, alleviate obesity-related complications such as knee pain, sleep apnea, and acid reflux.ucdavis
Emerging research explores their potential effects on the brain, including whether they can help reduce cravings for drugs, alcohol, and nicotine. At UC Davis Health, researchers are taking a holistic approach—examining GLP-1 drugs across multiple systems, from gastrointestinal and cardiovascular physiology to musculoskeletal and neurocognitive function.ucdavis
Side Effects and Safety Considerations
Common Side Effects
The most common side effects of both semaglutide and tirzepatide are gastrointestinal in nature and include nausea, vomiting, diarrhea, decreased appetite, stomach pain, constipation, dizziness, and fatigue. These side effects are usually mild to moderate and tend to diminish with continued treatment. Taking the medication with food can help minimize gastrointestinal issues.medicinecontact+1
In clinical trials, tirzepatide showed higher rates of certain gastrointestinal side effects like nausea, vomiting, and diarrhea compared to semaglutide. The rate of people stopping treatment due to side effects was also higher with tirzepatide.medicinecontact+1
Serious Side Effects
More severe possible side effects of both medications include vision changes, pancreatitis, kidney failure (with rare cases of kidney injury reported with semaglutide), gallbladder issues, and allergic reactions. Tirzepatide may cause vision changes particularly among those with diabetic retinopathy, while semaglutide is not known to impact vision. Anaphylaxis appears more common with tirzepatide.healthline+1
Both medications carry boxed warnings—the most serious warning from the FDA—about the risk of thyroid C-cell tumors. Despite these promising developments, GLP-1 therapies remain relatively new, with the first agent approved in 2005 for type 2 diabetes, and ongoing studies continue to examine their broader applications, long-term benefits, and safety profile.healthline+1
Healthcare System Impact
The explosion in GLP-1 use is creating significant downstream effects on healthcare systems. These medications have been associated with significant increases in health care premiums expected in 2026. One in eight adults currently takes these medications, and with oral versions becoming available and government deals slashing prices from $1,000+ to $50 copays, usage is expected to surge.ajmc+1
With continued studies of potential new uses for these drugs, broader and better insurance coverage is anticipated in 2026 and beyond. However, healthcare providers emphasize that GLP-1 medications necessitate ongoing clinical monitoring and must integrate into comprehensive treatment plans that include lifestyle adjustments.npr+1
Important Considerations for Patients
Weight management using GLP-1 medications is typically a prolonged process, with clinical trials assessing outcomes over 64 to 72 weeks. Patients must be ready for medication-supported interventions under medical supervision and able to commit to long-term treatment protocols. As the weight loss season commences, maintaining realistic expectations is more beneficial than accepting overblown promises.finance.yahoo
Compounded versions of these drugs lack FDA quality oversight and clinical safety data, raising risks around inappropriate use, uncertain side effects, unsafe dosing, and possible impurities. FDA-approved brands like Ozempic, Wegovy, Mounjaro, and Zepbound are safer options.medicinecontact

-(2).png)
Comments 0
No comments yet
Be the first to share your thoughts!
Leave a Comment